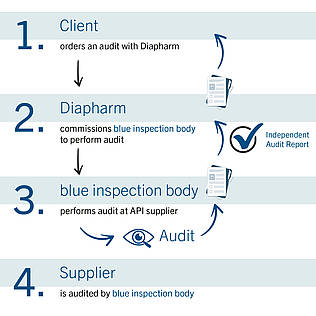
Diapharm Global Audit Solutions takes care of your customer requests for audits at your sites. Customer-Customized requests are coordinated by our team.
Our Global Audit Solutions team organizes the audits you require, for example:
APIs, Excipients, Food, GMP / GDP
For an overview of our audits in planning worldwide, please contact us! audits(at)diapharm.de
This is a list of active pharmaceutical ingredient (API) manufacturing sites worldwide, compiled by the Diapharm’s Global Audit Solution Team.
| Company | Site | Country | |
|---|---|---|---|
| Aarti Industries Limited | Tarapur | India | |
| ACS Dobfar | Vimercate | Italy | |
| Aesica Pharmaceuticals | Cramligton | UK | |
| Ajinomoto | Raleigh | USA | |
| Ajinomoto Omnichem | Wetteren / Louvian-La-Neuve | Belgium | |
| Akzo Nobel | Herkenbosch | Netherlands | |
| Aland (Jiangsu) | Jingjiang | China | |
| Alchem | Faridabad | India | |
| Alembic | Panelav | India | |
| Alkaloida | Tiszavasvári | Hungary | |
| Alkaloids | Telangana | India | |
| Amoli | Vapi | India | |
| AMRI | Maharashtra | India | |
| AMSA | Como | Italy | |
| Amsal | Ankleshwar | India | |
| Andhra Sugars | Tanuku | India | |
| Antibiotice | Iasi | Romania | |
| Antibióticos de León | León | Spain | |
| API Corporation | Fukuoka | Japan | |
| Apicore | Gujarat | India | |
| Arch Pharmalabs | Sagaon | India | |
| Archimica | Deeside Flintshire | UK | |
| Aspen | Moleneind | Netherlands | |
| Aspen | Diosite | Netherlands | |
| Auctus Pharma | Andhra Pradesh | India | |
| Avesta Pharma | Maharashtra | India | |
| Azico Biophore | Telangana | India |